To perform an acid–base titration step-by-step, start by understanding the basic principles of neutralization and choosing the appropriate method for your sample. Carefully prepare and standardize your titrant solution, set up your apparatus correctly, and select a suitable indicator for clear endpoint detection. Throughout the process, analyze your titration curve, calculate concentrations accurately, and troubleshoot common issues. If you explore these details further, you’ll gain confidence in executing precise titrations across various applications.
Key Takeaways
- Understand the chemical reaction and select an appropriate indicator based on titration type.
- Prepare solutions accurately, including standardizing the titrant against a primary standard.
- Set up the apparatus correctly, ensuring proper burette and pipette calibration and elimination of air bubbles.
- Perform the titration slowly near the endpoint, carefully observing color change and recording initial and final volumes.
- Analyze data by calculating molarity, verify reproducibility with multiple trials, and troubleshoot common issues like overshooting.
Understanding the Basics of Acid-Base Titrations

Have you ever wondered how chemists determine the concentration of an unknown acid or base? It’s done through acid-base titrations, which involve neutralizing the unknown solution with a titrant of known concentration.
You add the titrant gradually from a burette into the analyte until complete neutralization occurs, producing water and a salt. The goal is to find the exact volume of titrant needed, which lets you calculate the analyte’s concentration.
Key terms include the titrant, analyte, equivalence point, and indicator. The indicator signals when the reaction is complete through a color change, helping you identify the endpoint. Precise measurement and careful observation are essential for accurate results. Content quality and top‑ical authority are critical in ensuring reliable and credible experimental outcomes. This process is crucial across fields like pharmaceuticals, environmental testing, and industrial quality control. Additionally, understanding the chemical principles behind titrations can improve the accuracy and reliability of your measurements. Recognizing the importance of safe laboratory practices can also help avoid errors and ensure accurate titration results.
Types of Titration: Which One Fits Your Sample?

Choosing the right type of titration depends on the properties of your sample, as different methods suit specific acid-base characteristics. For samples with strong acids or bases, a straightforward titration with a sharp endpoint near pH 7 works best, often using indicator titrations. If your sample contains a weak acid or base, potentiometric titrations or carefully selected indicators provide more accurate results due to gradual pH changes. Complex or colored samples are better analyzed with instrumental methods like pH electrodes, which avoid misreading caused by color interference. Additionally, samples prone to CO2 interference should be pre-treated by boiling to eliminate dissolved carbon dioxide. Research supports matching your sample’s acidity strength and composition to the appropriate titration technique ensures reliable and precise measurements. Understanding sample properties can help determine the most suitable titration method for accurate analysis. Moreover, selecting the proper analytical method ensures your results are both accurate and reproducible across different sample types.
Preparing and Standardizing Your Titrant Solution

To guarantee accurate titrations, you must carefully prepare and standardize your titrant solution. Begin by calculating the required mass of your primary standard using the formula (m = c times V times M), ensuring you use an analytical balance with ±0.0001 g accuracy. Check the operating hours of your local supplier or store to purchase necessary chemicals at the right time. Transfer the solid quantitatively into a volumetric flask to prevent loss, and avoid hygroscopic substances unless stored properly. Dissolve the standard in minimal water, swirl gently, and rinse the vessel repeatedly to recover all solute. It is also important to ensure the purity of chemicals to achieve precise results. Additionally, verifying the accuracy of measurements is essential for reliable titration outcomes. Fill the burette with the titrant after rinsing it thoroughly, check for air bubbles, and record the initial volume precisely. Standardize against a certified primary standard by titrating in triplicate, calculating the molarity from the average volume, and applying blank corrections if necessary. Label and store the titrant properly for future use.
Setting Up the Titration Apparatus Correctly

Setting up your titration apparatus correctly is essential for obtaining accurate results. First, fix the burette vertically on a stable stand using a clamp to prevent movement. Position it above the Erlenmeyer flask containing the analyte, which should sit on a white tile or paper to improve visibility of color changes. Before filling, close the stopcock, then use a funnel to carefully fill the burette slightly above zero. Open the stopcock to drain out air bubbles, and wipe the outside with a clean tissue. Record the initial volume precisely at eye level. Proper instrument calibration ensures measurement accuracy throughout the titration process. Transfer a measured analyte volume into the flask using a pipette, rinse it beforehand, and add a few drops of the indicator. Ensure steady positioning and gentle swirling or magnetic stirring for thorough mixing.
Choosing the Right Indicator for Accurate Endpoint Detection

Selecting the appropriate indicator is key to accurately detecting the endpoint of a titration. You need to match the indicator’s pH transition range with the equivalence point of your reaction.
Choosing the right indicator ensures accurate titration endpoints and reliable results.
For strong acid-strong base titrations, where the pH at equivalence is near 7, bromothymol blue works well.
For strong acid-weak base titrations, with pH below 7, methyl orange is suitable.
For weak acid-strong base titrations, with pH above 7, phenolphthalein is ideal.
Using an indicator with a transition range that doesn’t overlap the equivalence point can cause false readings.
Always test the indicator beforehand to guarantee a clear, sharp color change.
Keep lighting consistent and avoid excess indicator, which might shift the endpoint.
Proper indicator choice ensures precise, reliable results.
Performing the Titration: Step-by-Step Procedure

Wondering how to perform a titration accurately? First, prepare your equipment and solutions.
Fill a clean burette with the titrant, ensuring no air bubbles are in the nozzle, and rinse all glassware with deionized water.
Use a volumetric pipette to measure a fixed volume of the analyte into a clean conical flask, then add a few drops of the chosen indicator.
Record the initial burette volume at eye level.
Slowly open the tap, swirling constantly to mix, and gradually increase the flow as the color change nears.
When the indicator signals the endpoint, add titrant dropwise, stopping immediately.
Record the final volume, repeat for concordant results, and calculate the titration volume.
Finally, clean all equipment and store properly for future use.
Analyzing the Titration Curve and Identifying the Equivalence Point

Analyzing the titration curve allows you to understand how the pH of a solution changes as you add titrant, providing valuable insights into the reaction’s progress.
As you observe the curve, look for the steepest part, which indicates the equivalence point where acids and bases are fully neutralized. In strong acid-strong base titrations, this point appears as a sharp vertical rise at pH 7.
However, for weak acids or bases, the pH at the equivalence point shifts, creating curves that slope more gradually. The curve’s shape helps you distinguish the equivalence point from the endpoint, which may differ slightly.
Recognizing these features ensures you accurately identify when the reaction reaches completion, enabling precise determination of the analyte’s concentration.
Calculating Concentrations and Interpreting Results

Understanding how to calculate concentrations during titrations is essential for determining the unknown analyte’s amount accurately.
First, identify the reaction’s balanced equation to understand the mole ratio.
Use the known concentration and volume of the titrant to calculate its moles.
Then, apply the mole ratio to find the moles of the unknown acid or base.
Divide this by its volume to determine its molarity.
Keep in mind, if the reaction ratio isn’t 1:1, adjust calculations using the appropriate coefficients.
Accurate volume measurements are critical, as errors can skew results.
Correct interpretation involves observing indicators to pinpoint the equivalence point and considering potential measurement errors.
Multiple trials help verify consistency, ensuring your calculated concentrations are reliable and reflect true chemical behavior.
Troubleshooting Common Issues in Titration Experiments

Accurate titration results depend on proper technique and awareness of potential issues that can arise during experiments. Temperature fluctuations can change acid and base dissociation, skewing results. Contamination from impurities affects solution concentrations, leading to inaccuracies. Incorrect calibration of measuring devices causes volume errors. Air exposure introduces CO₂, altering pH levels. High humidity can interfere with electronic equipment.
Troubleshooting technique issues involves controlling titration speed to prevent overshoot and ensuring thorough mixing for accurate endpoint detection. Choosing the right indicator and conducting titrations within its ideal pH range also improve precision. Be mindful of incompatible reagents, side reactions, and incomplete neutralization, which can distort results.
Regularly maintain and calibrate instruments and electrodes, and handle chemicals carefully to avoid safety hazards and ensure reliable outcomes.
Practical Applications of Acid-Base Titrations in Industry and Research
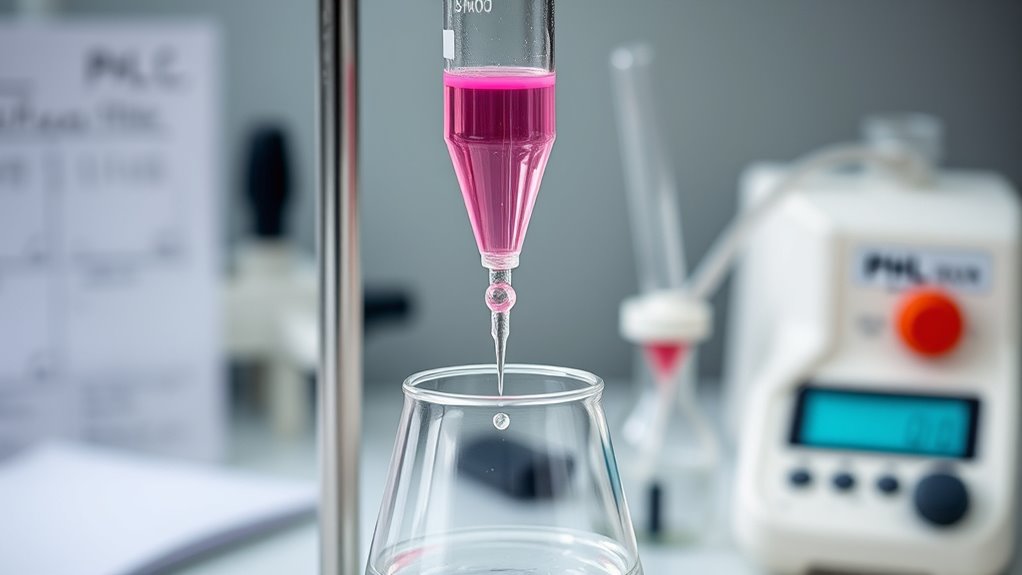
Acid-base titrations play a vital role across various industries and research settings by providing precise measurements essential for quality control, process optimization, and scientific investigation.
Acid-base titrations are essential for precise measurements in industry and research applications.
In the pharmaceutical industry, you use titrations to verify API concentrations, develop formulations with proper pH for stability, and analyze medicine purity. They also help control production by monitoring pH during synthesis.
In chemical manufacturing, titrations optimize yields, control acidity, and guarantee product quality in large-scale processes like fertilizer production.
The food industry relies on titrations to maintain flavor, safety, and shelf-life by controlling pH in fermented foods and beverages.
Environmental research uses titrations to assess water quality, monitor pollution, and evaluate ecological impacts.
In R&D, you determine compound concentrations, study reaction mechanisms, and validate experimental conditions efficiently.
Frequently Asked Questions
How Do I Select an Appropriate Indicator for My Titration?
When selecting an indicator, you need to match its pH shift range with your titration’s equivalence point.
Choose one with a sharp, clear color change that’s easy to see. Make certain it’s chemically stable and inert, so it doesn’t interfere with your reaction.
Also, consider cost and availability. By aligning these factors, you guarantee accurate endpoint detection and reliable results in your titration.
What Are Common Signs of Endpoint Detection Errors?
Think of endpoint detection as your security guard—when it’s not alert or misses cues, problems arise. You might notice incomplete coverage, missed threats, or too many false alarms.
Alerts may overwhelm you, automation could lag, or responses mightn’t match your policies. Compatibility issues with legacy systems or poor integration can leave gaps.
Recognizing these signs helps you fix issues before breaches happen, keeping your defenses strong and vigilant.
How Does Temperature Affect Titration Accuracy?
Temperature impacts titration accuracy by speeding up reaction rates, which can cause rapid endpoint shifts and overestimation of titrant volume if you’re not careful. It also alters pH and equilibrium, especially in weak electrolytes, leading to shifted titration curves.
Additionally, thermal expansion affects volume measurements, and high temperatures degrade indicators or reagents, increasing errors. To guarantee precision, control temperature closely and adjust measurements for thermal effects.
When Should I Repeat Titrations for Reliable Results?
You should repeat titrations whenever you notice inconsistent results or discrepancies between trials. Repeating helps confirm accuracy, identify errors, and refine your technique.
It’s crucial to do multiple trials under the same conditions, especially if reagent quality, equipment calibration, or environmental factors vary. Repetition ensures your data is reliable, precise, and can be confidently used for analysis or sharing with others.
How Can I Minimize Titrant Overshoot Near the Equivalence Point?
Ever wonder how to prevent overshooting the endpoint? You should slow down titrant addition as you near the equivalence point, using finer control with the buret stopcock. Switch to smaller volume increments and monitor pH closely with a meter or indicator.
Practice delivering precise volumes, calibrate your equipment, and recognize the steep pH change. These steps help you stop exactly at the equivalence point, ensuring accurate and reliable titration results.
Conclusion
Mastering acid-base titrations is like revealing a secret code in chemistry. With careful preparation and attention to detail, you’ll confidently navigate from theory to practice. Remember, every successful titration brings you closer to understanding the delicate balance of acids and bases—much like discovering the harmony in a symphony. Keep practicing, and you’ll turn complex reactions into your greatest scientific achievement, proving that precision and patience truly lead to mastery.









