CRISPR gene editing combines biology and chemistry by using the Cas9 enzyme guided by RNA to target specific DNA sequences next to PAM sites. The enzyme creates double-strand breaks, which are repaired either by error-prone NHEJ or precise HDR pathways. Chemical modifications to guide RNAs enhance stability and reduce off-target effects. Advances like base editing rely on chemical tweaks for precise changes. Exploring this chemistry-rich process reveals how scientists are perfecting gene edits; if you continue, you’ll discover even more.
Key Takeaways
- Cas9 enzyme uses guide RNA to locate and bind to specific DNA sequences adjacent to PAM sites for targeted editing.
- The guide RNA’s chemical modifications enhance stability, reduce degradation, and improve editing accuracy.
- Cas9 induces double-strand breaks at precise locations, initiating DNA repair pathways like NHEJ and HDR.
- DNA repair mechanisms determine whether gene edits result in deletions, insertions, or precise modifications.
- Engineering Cas9 variants and base editors optimize specificity and enable reversible, mutation-specific gene modifications.
Understanding the CRISPR-Cas9 Mechanism

Understanding the CRISPR-Cas9 mechanism begins with recognizing its key components and how they work together. You have the Cas9 enzyme, a nuclease from *Streptococcus pyogenes*, which introduces double-strand breaks (DSBs) in DNA. Guided by a specially designed guide RNA (gRNA), Cas9 locates its target sequence by base pairing with a 20-nucleotide segment of DNA. The process also involves the PAM sequence, a short motif like NGG, which Cas9 scans for to confirm the target. Once recognized, Cas9 undergoes a structural change, unwinding the DNA to form an R-loop. The enzyme then cleaves both DNA strands—targeted by the HNH domain and the RuvC domain—creating a precise DSB that activates the cell’s repair pathways. The PAM sequence is critical for target recognition and specificity.
The Role of Guide RNA and PAM Sites

Guide RNA (gRNA) and PAM sites are essential components that determine the precision and effectiveness of the CRISPR-Cas9 system. The gRNA includes a 20-nucleotide sequence matching your target DNA and a scaffold for Cas9 binding. It guides Cas9 to the precise DNA location. The PAM site, usually NGG for Cas9, must be adjacent to the target for Cas9 to cut effectively. Without the PAM, Cas9 won’t bind or cleave the DNA, limiting target options. Variants like Cas12 recognize different PAM sequences, broadening possibilities. Here’s a visual overview:
| Component | Function | Importance |
|---|---|---|
| Guide RNA (gRNA) | Recognizes target DNA | Ensures specificity |
| PAM Site | Adjacent to target DNA | Required for Cas9 binding |
| Cas9 Enzyme | Creates DNA double strand break | Executes the cut |
How Cas9 Enzyme Finds and Cuts DNA

How does Cas9 enzyme locate and cut specific DNA sequences so efficiently? Cas9 forms a complex with guide RNA, which helps it scan the DNA for a matching sequence. As it moves along, Cas9 causes local melting of the DNA strands, allowing it to check for a perfect match with the guide RNA. This scanning process is highly dynamic, involving multiple conformational changes in the enzyme. Once it recognizes the correct sequence next to a PAM site—usually NGG—it becomes activated. The enzyme then creates a double-stranded break by cleaving both DNA strands. Cas9 contains HNH and RuvC-like domains that cut complementary and non-complementary strands, respectively. Dreams and subconscious processing can sometimes reveal patterns or symbols that relate to biological processes or challenges. This process is rapid and precise, enabling CRISPR to target specific genes quickly and efficiently for editing.
Repair Pathways in Gene Editing: NHEJ and HDR
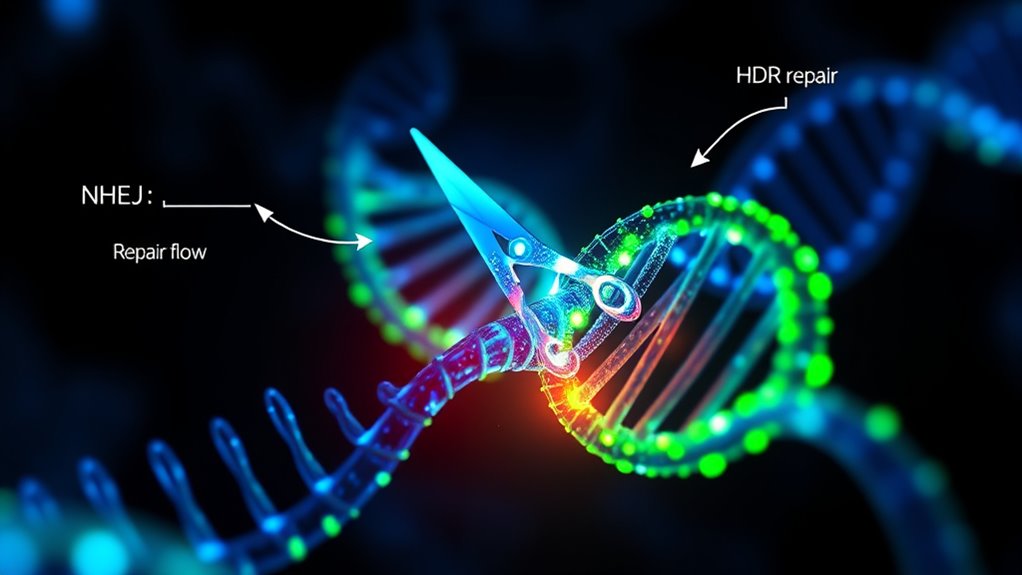
Once Cas9 makes a precise cut in the DNA, the cell must repair the break to restore genetic integrity. You have two main options: Non-Homologous End Joining (NHEJ) and Homology-Directed Repair (HDR). NHEJ is fast and active throughout most of the cell cycle, but it’s error-prone, often causing insertions or deletions—great for knocking out genes. HDR is more accurate because it uses a template to guide repair, making it ideal for precise modifications like gene knock-ins, but it’s less efficient and restricted to the S and G2 phases. While NHEJ dominates in mammalian cells, HDR’s accuracy is vital for clinical applications. By manipulating these pathways—such as inhibiting NHEJ—you can enhance precise editing and achieve your genetic modification goals more effectively. Understanding how to control these pathways is crucial for optimizing gene editing outcomes. Additionally, advances in DNA repair modulation are opening new possibilities for more efficient and precise gene therapies.
Chemical Modifications to Enhance CRISPR Efficiency

Chemical modifications of guide RNAs (gRNAs) can substantially boost CRISPR editing efficiency, especially in primary mammalian cells and in vivo applications. By adding phosphorothioate linkages to the backbone, you improve resistance to nucleases, increasing gRNA stability and activity.
Incorporating 2′-O-methyl (2′-OMe) and 2′-fluoro (2′-F) modifications enhances degradation resistance and target binding. Terminal modifications like 2′-O-methyl-3′-phosphorothioate prevent exonuclease degradation, prolonging gRNA lifespan and boosting on-target editing with minimal off-target effects. These chemical modifications are also critical for reducing immunogenicity, supporting safer therapeutic applications.
These chemical tweaks support highly active RNP platforms, reduce cytotoxicity, and improve specificity by fine-tuning Cas9 binding. Ultimately, chemically optimized gRNAs are essential for maximizing gene editing efficiency in complex in vivo environments, especially when targeting primary cells. Such modifications also assist in reducing immune responses, further enhancing the safety of in vivo applications.
The Chemistry Behind Base and Prime Editing Technologies
Base and prime editing technologies rely on precisely engineered chemical reactions to modify DNA without causing double-strand breaks. In base editing, a modified CRISPR-Cas9 fused to a deaminase enzyme chemically changes specific bases. Cytosine base editors (CBEs) convert C to T by removing an amino group, while adenine base editors (ABEs) transform A into G through deamination. These reactions enable targeted base-pair substitutions, with newer editors expanding possible changes. Base editing was first introduced in 2016, marking a significant advancement in genome editing technology. Prime editing uses a Cas9 nickase fused to reverse transcriptase, guided by a pegRNA that encodes the desired edit. The nickase creates a single-strand break, allowing the reverse transcriptase to synthesize the new DNA sequence directly onto the target strand. Both methods achieve precise edits by harnessing chemical modifications, avoiding the risks associated with double-strand breaks. Chemical reactions are fundamental to the specificity and efficiency of these editing techniques, enabling precise genetic modifications without unintended damage.
Challenges in Delivery and Off-Target Effects

Delivering CRISPR components effectively to specific tissues remains one of the biggest obstacles in gene editing therapies. Targeting internal organs or crossing the blood-brain barrier adds complexity, making delivery even harder. Privacy and Consent Management plays a role in how data related to these therapies is handled and shared. Viral vectors like AAV and adenoviruses are common but can trigger immune responses, reducing efficiency. Nonviral vectors, such as liposomes, and physical methods are being explored to improve safety and delivery success. Off-target effects pose a major safety concern, as unintended genetic changes can cause cell damage or chromosomal issues. Advances like engineered Cas9 variants and base or prime editing aim to minimize these risks. Improving guide RNA design and delivery methods is essential for balancing efficiency, safety, and precision in gene editing therapies.
Future Innovations in CRISPR Chemistry

As researchers explore new frontiers in CRISPR technology, innovations in chemistry are expanding the toolkit for precise and versatile gene editing. New Cas proteins with unique properties increase targeting options, allowing edits in previously inaccessible cell types or organisms. Chemical modifications to guide RNAs improve stability and specificity, boosting efficiency. Chemically engineered Cas variants reduce off-target effects, enhancing safety. Introducing non-natural nucleotides and linkers provides precise control over activity and delivery. Base and epigenetic editors enable reversible and mutation-specific modifications without double-strand breaks, reducing risks. Combining CRISPR with diagnostic tools accelerates detection, while targeted therapies benefit from improved temporal regulation. Advances in trailer music techniques and sound design are also supporting innovative storytelling in media. These advancements open doors for breakthroughs in medicine, agriculture, and environmental science.
| Innovation Area | Key Features |
|---|---|
| New Cas Variants | Broader targeting and functions |
| Chemical Guides | Increased stability and specificity |
| Engineered Cas | Reduced off-target effects |
| Base/Epigenetic | Precise, reversible modifications |
| Diagnostics & Therapy | Enhanced detection and control |
Frequently Asked Questions
How Does CRISPR Distinguish Between Similar DNA Sequences?
You might wonder how CRISPR tells similar DNA sequences apart. It uses multiple checks: PAM sequences ensure it targets only foreign DNA, while crRNA spacer matching confirms precise hybridization.
Structural changes during DNA unwinding and kinetic proofreading help detect mismatches. These combined mechanisms guarantee CRISPR mainly cleaves intended targets, avoiding off-target effects, by verifying both the presence of PAMs and perfect sequence complementarity.
What Chemical Factors Influence Guide RNA Stability?
You should know that several chemical factors influence guide RNA stability. Incorporating modifications like 2’-O-methyl groups, 2’-fluoro substitutions, and locked nucleic acids enhances resistance to nucleases.
Proper buffer storage, avoiding RNase contamination, and controlling freeze-thaw cycles also help preserve stability.
Additionally, maintaining a desirable GC content between 40-80% ensures strong base pairing without forming disruptive secondary structures, ultimately supporting effective genome editing.
Can CRISPR Target Mitochondrial DNA Effectively?
You wonder if CRISPR can effectively target mitochondrial DNA. While promising, it faces challenges like delivering guide RNAs into mitochondria through their double membranes.
Smaller effectors like Cas12a help, but delivery remains a bottleneck. You need specialized signals and vectors, such as viral carriers, to improve mitochondrial import.
Despite obstacles, ongoing research shows potential for mitochondrial gene editing, especially for treating mitochondrial diseases by shifting heteroplasmy or eliminating mutant DNA.
What Are the Chemical Safety Concerns With CRISPR Components?
Think of CRISPR components as a double-edged sword—powerful yet risky. You should be aware of chemical safety concerns, like cytotoxicity from delivery reagents such as lipid nanoparticles or viral vectors.
Contamination or degradation during manufacturing can introduce harmful byproducts. Additionally, off-target interactions might cause unintended effects.
Guaranteeing reagent stability, purity, and biocompatibility is vital to avoid dangerous side effects and ensure safe, effective gene editing.
How Do Chemical Inhibitors Improve CRISPR Precision?
You can improve CRISPR precision by using chemical inhibitors that target DNA repair proteins like DNA-PK and PolΘ. These inhibitors shift repair pathways toward more accurate HDR, reducing unwanted mutations.
They also allow you to control Cas9 activity temporarily and reversibly, minimizing off-target effects.
Combining different inhibitors enhances editing accuracy and efficiency across various cell types, helping you achieve cleaner, more precise gene edits.
Conclusion
By mastering the chemistry behind CRISPR and gene editing, you open groundbreaking potential to transform medicine and agriculture. With each chemical tweak and innovation, you push the boundaries of what’s possible—turning science fiction into reality. As you continue to refine delivery methods and minimize off-target effects, you’re not just making small advances; you’re on the brink of rewriting the very fabric of life itself. The future of gene editing is brighter than you’ve ever imagined.