Discovering new antibiotics involves finding innovative ways to target bacteria while overcoming old challenges like resistance. Bacteria evolve quickly, making many existing drugs ineffective over time. Researchers explore natural products, which offer diverse structures and novel mechanisms. Advances in genomics and high-throughput screening speed up discovery but still face hurdles like complex extraction and ecological concerns. If you continue exploring, you’ll uncover how science is tackling these ongoing struggles with fresh strategies.
Key Takeaways
- Developing antibiotics with novel mechanisms can overcome existing bacterial resistance.
- Natural product screening remains vital for discovering structurally unique antibiotics.
- Resistance evolution necessitates continuous search for new targets and modes of action.
- Advances in genomics and high-throughput methods accelerate identification of promising compounds.
- Integrating sustainable practices ensures ethical, efficient exploration of natural sources for antibiotics.
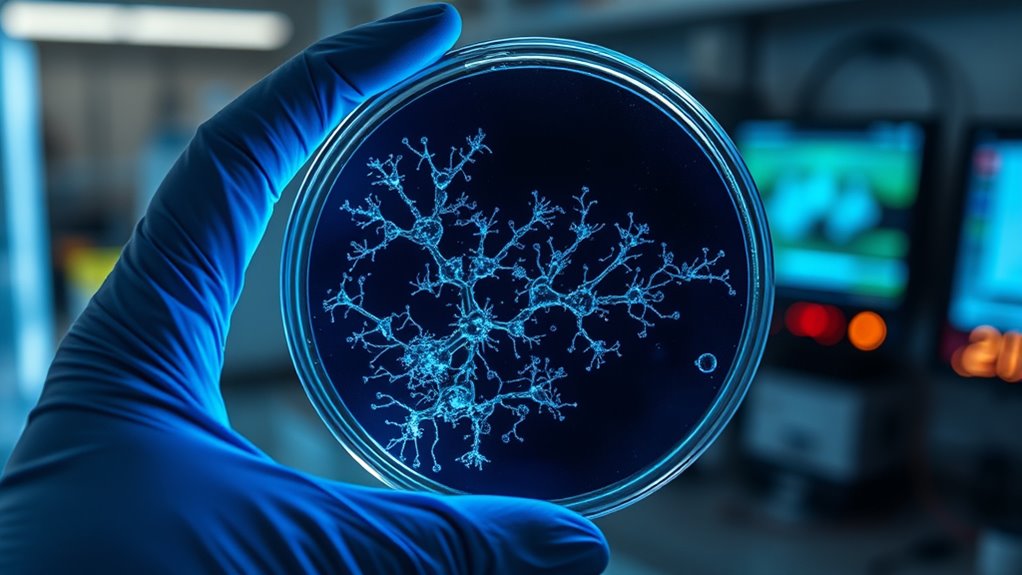
The discovery of antibiotics revolutionized medicine by providing powerful tools to fight bacterial infections that once claimed countless lives. However, as you delve deeper into antibiotic development, you quickly realize that bacterial resistance is one of the biggest hurdles. Bacteria evolve rapidly, often mutating or acquiring resistance genes that make them impervious to existing drugs. This ongoing arms race means that many antibiotics become ineffective over time, forcing scientists to seek new solutions constantly. One approach gaining renewed attention is natural product screening, which involves exploring diverse ecosystems for novel compounds produced by microorganisms, plants, and fungi. These natural products have historically been a rich source of antibiotics, and by focusing on them again, you might uncover new mechanisms of action that bacteria haven’t encountered before.
Natural product screening revives antibiotic discovery by exploring ecosystems for novel, resistance-breaking compounds from microorganisms, plants, and fungi.
When you engage in natural product screening, you’re essentially tapping into a vast reservoir of chemical diversity that evolution has refined over millions of years. Many antibiotics in use today originated from natural sources, such as penicillin from mold or streptomycin from soil bacteria. These compounds often have complex structures that are difficult to synthesize artificially, making natural extraction and screening essential. By isolating and testing these compounds against resistant bacterial strains, you can identify potential antibiotics with unique modes of action, which is crucial in overcoming bacterial resistance. Moreover, natural products may also serve as scaffolds for developing synthetic derivatives with improved potency and reduced resistance development.
Despite its promise, natural product screening isn’t without challenges. The process can be time-consuming and labor-intensive, often requiring sophisticated techniques to extract, purify, and analyze compounds. Many natural sources produce a mixture of substances, and identifying the active component can be like finding a needle in a haystack. Additionally, some natural compounds may have limited availability or be difficult to produce in large quantities. Nonetheless, advances in genomics, metabolomics, and high-throughput screening are helping you overcome these obstacles, enabling faster identification of promising candidates. As you explore these natural reservoirs, it’s vital to consider the ecological and ethical implications of harvesting resources, ensuring sustainable practices.
Furthermore, the integration of Kia Tuning techniques, such as engine remapping or suspension upgrades, into your research process can metaphorically enhance your ability to “tune” your approach to discovering effective antibiotics, making the process more efficient and targeted. In your quest for new antibiotics, understanding bacterial resistance mechanisms is essential. You need to stay ahead by discovering drugs that can bypass or inhibit resistance pathways. Natural product screening offers a promising route, providing chemical diversity and novel mechanisms that synthetic libraries might lack. By combining traditional microbiology with cutting-edge technology, you can contribute to a future where bacterial resistance is managed more effectively. The challenge remains significant, but with persistence and innovation, new antibiotics born from nature’s own arsenal could once again turn the tide in fighting resistant bacteria.
Frequently Asked Questions
How Do Bacteria Develop Resistance to New Antibiotics?
You develop resistance to new antibiotics through various methods, like horizontal gene transfer, which allows bacteria to acquire resistance genes from other microbes. Additionally, bacteria activate efflux pumps that expel antibiotics before they can work effectively. These strategies enable bacteria to adapt rapidly, making it harder for new antibiotics to stay effective. Staying aware of these mechanisms helps in developing better drugs and combating resistance more effectively.
What Are the Future Prospects for Alternative Antimicrobial Therapies?
You can look forward to alternative antimicrobial therapies like phage therapy and antimicrobial peptides gaining traction. Phage therapy uses viruses that target bacteria specifically, reducing resistance risks, while antimicrobial peptides disrupt bacterial membranes effectively. As research advances, these options could become essential tools in fighting resistant infections, offering targeted, sustainable solutions. Embracing these therapies may revolutionize how you combat bacterial threats in the future.
How Do Regulatory Agencies Evaluate New Antibiotic Approvals?
Imagine steering a maze filled with regulatory hurdles—that’s what approval processes feel like for new antibiotics. Regulatory agencies carefully scrutinize your data, ensuring safety and efficacy before granting approval. They evaluate clinical trial results, manufacturing quality, and potential risks. This meticulous process acts as a gatekeeper, balancing the urgent need for new antibiotics with the responsibility to protect public health, guiding your innovation safely from lab to patient.
Can Natural Products Be Rediscovered for Antibiotic Use?
Yes, you can rediscover natural products for antibiotic use by exploring their vast diversity. Natural product diversity offers a rich source of novel compounds, and understanding biosynthetic pathways helps you identify and engineer these molecules for improved activity. By tapping into these pathways, you can uncover new antibiotics or enhance existing ones, making natural products a valuable resource in combating resistant bacteria and addressing old challenges in antibiotic discovery.
What Role Do Microbiomes Play in Antibiotic Effectiveness?
Your microbiome diversity influences how effective antibiotics are because it affects host microbe interactions. A diverse microbiome can help resist harmful bacteria, making antibiotics more effective, while a less diverse one might allow resistant strains to flourish. You can support this by maintaining a healthy gut, which promotes beneficial host microbe interactions that enhance antibiotic action and reduce side effects. Understanding this relationship helps optimize treatment outcomes.
Conclusion
As you stand at the crossroads of discovery, remember that antibiotics are the keys guarding our future. The battle against resistance is like fighting a relentless storm, but each new mechanism you uncover is a beacon of hope. Don’t let the flame of innovation flicker out—keep pushing forward. Your efforts are the lighthouse guiding us through turbulent waters, illuminating the path toward a world where medicine and resilience shine bright against the darkness of old challenges.









